Clinical Trials Feasibility Platform – An Intelligent, Data Driven Analytics Platform
Introduction
The life sciences industry is struggling to bring products faster to the market at a reduced cost. The rising cost of drug development and the impact of the pandemic have led life sciences organizations to reinvent the conducting of clinical trials. The success of clinical trials depends on the right investigator and site selection, leading to the proper patient recruitment. 80% of trials sites fail to meet enrollment timelines, and 45% of trials cannot meet completion deadlines. Approximately 70% of potential participants/subjects live more than 2 hours away from their nearest study center in traditional clinical trials. The average dropout rate observed across all clinical trials is 30%. The Covid-19 Pandemic has shifted the focus of clinical trials from site-centric to decentralized and patient-centric trials. Regulatory bodies are now emphasizing exploring novel technologies to accelerate and continue clinical trials. These changes have caused organizations to analyze various data sources that produce large, diverse, and complex information, which can explain, influence, and provide insights to make informed decisions to reduce costs and time to market for a drug.
However, challenges such as siloed data from multiple sources (internal & external), recruitment failures, and a complex environment of various technology solutions add to a pharmaceutical organization’s woes.
A digital, unified, data-driven integrated clinical analytics platform can help life sciences organizations towards effective protocol development, feasibility evaluations, pre-study, during-the-study and post-study activities. It can also help manage the organization's portfolio with the help of a human-centered user interface.
Problems worth Solving
Conducting clinical trials requires significant planning, forecasting, and cooperation among several stakeholders for trial feasibility, and this is the most crucial step in conducting clinical trials. Clinical research infrastructure, medical and clinical supplies, the support system of informatics, and workforces are critical for conducting tests and completing them on time and within a budget. The average cost of releasing a drug to market is estimated to be ~2 Billion USD. Slow adoption of technologies, siloed data, and operational dependencies increase the cost of trials by at least 20%. Let us look at some of the common challenges faced by the stakeholders involved in the clinical trials.
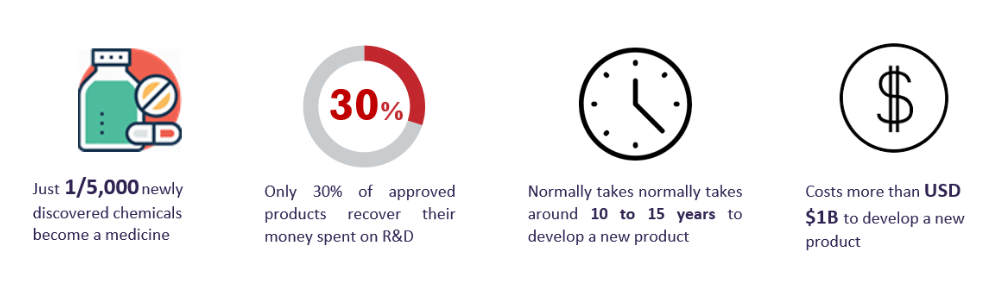
Competitor Drug Information
A holistic understanding of the competitive landscape, competitor’s drug and asset profiles can help predict critical metrics and enable a sponsor to have a proper drug development strategy.
The US pharmaceutical industry was always a highly competitive market space and has now become even more so. In recent years, the market has become even more flooded with both branded and generic products. Major companies compete with each other, and practically all of them are active in producing drugs and conducting R&D processes.
Protocol Design
Conducting clinical trials requires intensive planning and prompt execution to achieve the outcomes on time. Although technological innovations have reduced pre-clinical development phases, the clinical testing phase has not made significant progress compared to the technologies and costs involved.
As the regulatory landscape evolves, the requirement for safety and efficacy becomes more stringent. Therefore life sciences organizations are looking for innovative approaches to shorten the duration of the clinical phases of drug research and development and be in a compliant state. Primary research, social media listening to gather patient perspectives, clinical forecasting are the keys to ensuring that a protocol does not pose an unforeseen emotional or logistical hurdle for patient participation. Successful clinical trials require improved patient-centric protocol designs.
Selection of Right Geography or Country
Conducting clinical trial feasibility is one of the most crucial steps in clinical trial conduct. Identifying the correct country, assessing internal and environmental capacity in a particular geographical location, and aligning the clinical trial in terms of study design, the dosage of investigational product, disease prevalence and incidence, patient types, and patient availability is of utmost importance. Patient enrollment, lack of infrastructure at potential site/trial center, absence of relevant investigators, availability of critical support resources, departments, and personnel (IRB, local lab, diagnostics, etc.) can hamper the launch of a clinical trial in a new location. Initial projections of patient recruitment need to be precise to avoid the financial, clinical, and ethical costs of trial extensions or failures. However, estimation of recruitment rates is challenging and often poorly executed, if attempted at all. Previously used Protocol designs for one geography may not guarantee success in another. Moreover, a flawed Protocol design may result in enrollment difficulties, overshot timelines, escalating costs, and higher termination rates.
Patient Recruitment Strategies
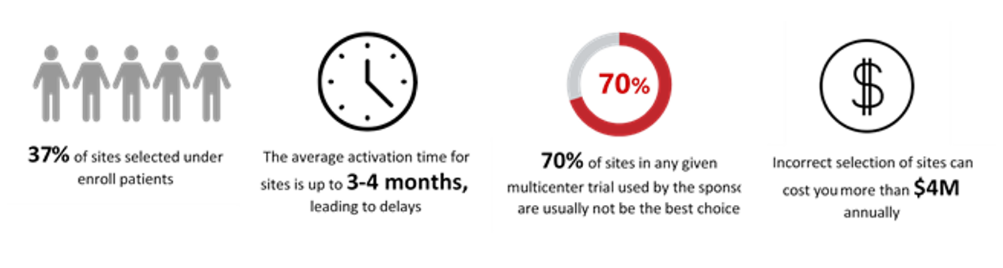
Patient recruitment is one of the primary reasons that cause delays in completing clinical trials on time. 80% of trials sites fail to meet enrollment timelines. 11% of sites fail to enroll a single patient, and 85% of clinical trials fail to retain enough patients. 20% of investigators do not register a single patient. 33% of investigators enroll only 5% of evaluable patients. Hence, identifying the right investigators and sites has become paramount for recruiting suitable patients for the success of clinical trials.

(Source: ncbi.nlm.nih.gov)
Site & Investigator Performance
Accelerated study start-up is essential for the timely execution of clinical trial operations. While advancements like central Institutional Review Board (IRB) review, adaptive trial designs, and the application of electronic systems have led to increased efficiencies across the clinical trial enterprise, the overall start-up phase of a clinical trial continues to be the primary source of backlog.
(Source: ncbi.nlm.nih.gov: clinicaltrials.gov)
Further, research suggests that only 1/3rd of the investigators in a trial consistently enroll patients, and 20% do not enroll any patients at all. Investigators usually overestimate the number of patients they expect to recruit based on the survey data, sometimes as much as 75%, erroneously based on projected enrollment capacity.
Data Enablement
Most clinical trials planning depends on internal databases, which house knowledge based on previous studies. Therefore using this information may not be the best choice. The data provided by internal databases do not consider the latest real-world data, which provide more accurate information trials can use.
Computers, mobile devices, wearables, and other biosensors have become extremely capable of gathering and storing huge amounts of health-related data. This data can allow sponsors to better design and conduct clinical trials to address previously thought impossible questions.
At an initial glance, supplementing additional data fields into business intelligence systems can be a costly affair. Therefore, researchers often deprioritize it, ignoring the long-term benefits of integrated big data analysis. Organizations need to create a basis to collect, store, organize and interpret data efficiently to exploit the full potential of data. Further, labor-intensive and time-consuming research and tracking databases changes also add to the lack of efficiency.
Role of Contract Research Organizations
Sponsors may seek the support of Contract Research Organizations (CRO) and other service providers for clinical trials. With a global outreach and the preparedness to move forward, CROs can greatly benefit sponsors in the clinical trials process. However, delegating duties and functions to CROs does have challenges. As there are no guarantees of timely completion of the tasks related to clinical development, the sponsors often have to bear the consequences like delayed completion of studies and increased development cost. By the nature of the arrangement, sponsors do not get involved with the day-to-day operations of the CRO, which often disrupts the communication flow. Thus, it can be difficult for sponsors to keep a tab on the processes conducted by the CROs. Along with the challenges mentioned above, the pandemic has added more complexity to the clinical development process. The industry is looking for near real-time visibility of studies conducted for better and faster decision-making.
The Changing Clinical Trial Models
Due to the Covid – 19 pandemic, companies have shifted the focus of clinical development to manage development activities remotely, perhaps from the patient’s home, instead of sites.
Integrated, intelligent, and data-driven "one-stop-shop" analytics-based solutions are now in demand to support decentralized clinical trials. Organizations are currently looking for unified cloud platforms with additional support services, site networks, and data capabilities all in one place.
Advancement in technologies and pressure from regulators has caused companies to explore novel ways to accelerate clinical trials like leveraging cloud, IoT, big data analytics, and cognitive technologies, bringing innovation, automation, and digital experience. Although this new approach requires strong technical support and infrastructure, estimates predict that the overall costs will be significantly lower than the traditional clinical trials.

The geographic proximity of patients’ homes to research sites, number of site visits, the amount of time required to participate in a trial, and the pandemic are some of the reasons why patients are apprehensive about participating in a clinical trial. These factors impede patients from visiting study sites, receiving scheduled dosing, or attending necessary on-site screenings. The site staff can often access study records, collect patient lab samples or conduct patient interviews, limiting site and data monitoring. Ideally, decentralized clinical trials approaches are selected and homogenized into a clinical protocol long before recruitment begins during the study design phase. The COVID-19 crisis pushed the sponsors to consider alternative patient-centric approaches to ensure the continuity of trials.
Identifying patients within and beyond the site’s database has been essential to support site-based recruitment and patient engagement. These capabilities directly affect individual studies and overall delivery strategies. Optimizing patient enrollments by improving patient recruitment rates offers a clear advantage that reduces the time to launch.
Decentralized Trials
The Decentralized Clinical Trials (DCTs) model or the site‐less or virtual study model is a patient-centric trial aiming to eliminate the need for patients to travel to an investigational site. DCTs are a relatively new yet underutilized method of conducting clinical research taking full advantage of mobile applications, electronic monitoring devices, and online social engagements. This patient‐centric model is getting popular for helping to reduce the number of sites and study-staff, thus lowering operating costs, reducing patient dropout rates, and the time associated with site development and patient recruitment phase.
Cloud Adoption
Adoption of cloud technologies can revolutionize a pharmaceutical company’s clinical trial outcomes. The benefits of a centralized collaboration platform are clear to see during the protocol development phase. Archiving the latest document with the most recent amendments in one central location can circumvent the confusion that ensues when everyone is trying to keep track of the newest version on their own. A Centralized cloud management system can save time for life science organizations conducting clinical trials with complex logistics such as multiple sites enrollment and remotely monitored participants.
Cloud services facilitate data standardization, presentation, and visualization that help clinical research organizations and other partner organizations integrate, share, and analyze data from clinical trials, adhering to data security, compliance, and flexibility.
The scalability of cloud data management services can be swiftly modified to cater to drug testing on new groups and larger populations. In the years ahead, both pre-and post-approval trials will only expand in size and scope as more patients join in. With the right cloud-managed services partner, pharmaceutical companies and businesses will serve patients to enter and succeed in this promising new landscape for clinical trials.
Addressing the problem
There is a need for an intelligent, data-driven integrated analytics platform that leverages insights from a huge pool of external and internal data sources. This platform should also facilitate a sponsor with data-driven insights to effectively assess the investigator, site, and protocol feasibility during clinical trial planning. Sponsors also need a solution that performs a what-if analysis during the study and suggests mitigating strategies to avoid cost overruns and overshot timelines.
Cognitive technologies such as artificial intelligence (AI) and machine learning are evolving with a promise of solving current industry challenges. These challenges include faster marketing of new treatment solutions, accelerating clinical development activities, including clinical trial planning and risk management using a data-driven analytical approach to perform a feasibility analysis of clinical trials. The platform can also minimize time delays by identifying suitable patient pools, sites, investigators, and KOLs for the study. This platform can also mitigate risks across the trial process by objective analysis of earlier trials and patient enrollment data. By analyzing the competitor data, manufacturers can gain more visibility on the previous trials conducted at sites by various sponsors in specific therapeutic areas. Recent research suggests that AI and automation could cut the cost of drug development by as much as 70 percent.
The Solution
The solution implements a next-generation intelligent clinical trials feasibility AI platform with solid data, knowledge, and cognitive capabilities. The platform can facilitate sponsors with data-driven insights to effectively assess investigator, site, and protocol feasibility with harmonized internal and external data during the clinical trial planning phase. It can also enable sponsors to look beyond their internal databases by leveraging real-time insights from a vast pool of external data sources, identifying the risks, and making a go or no-go decision much earlier. Implementing this solution can result in fewer protocol amendments, providing a view of the best-performing sites & investigators, eliminating under or no patient enrollments sites and investigators, and rendering a bird’s eye view of the competitor trial landscape.
The platform can also analyze the data collected during the clinical study phases to provide a what-if analysis and suggests mitigation strategies to avoid extended budgets and timelines.
The platform derives its knowledge from a variety of ingested datasets that can provide real-time insights and facilitate data-driven decisions:
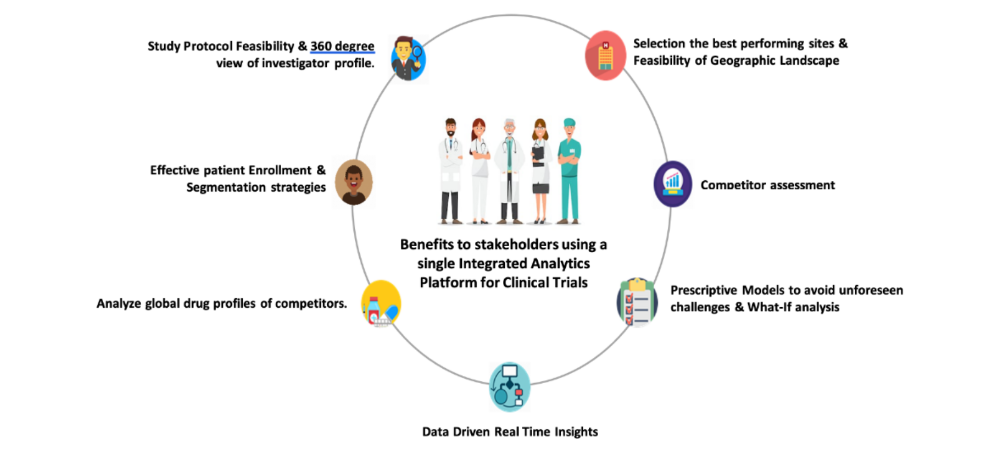
This platform will help sponsors with more accurate clinical trial planning and risk management strategies by using a data-driven analytical approach to feasibility analysis of clinical trials.
The benefits to the sponsors are:
The platform will help in identifying the right investigator and patient pools for the study. Assist in finding the suitable sites that are most likely to succeed in the study. This way, the customers avoid non-active and non-enrolling sites, thereby considerably reducing the associated trial costs and time.
The platform helps in the objective analyses of previous studies and the associated key investigators, sites, and collaborators to provide accurate go-ahead and mitigation strategies during the pre-study planning phases. It also assesses the data generated during the study phases to provide precise what-if analysis.
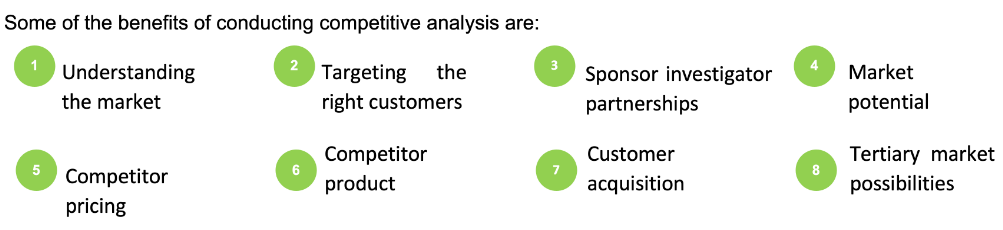
Using the platform, sponsors can gain information on the performed trials and correctly calculate the costs involved to make better decisions. The platform also provides a detailed competitor assessment, including an analysis of previous trials' success and failure rate, details of ongoing trials, sites, KOLs, CROs that competitors frequently collaborate with for their studies.
Key Takeaways
Conclusion
Data is a new currency; drug manufacturers can accelerate drug development lifecycle and gain enormous information and insights by leveraging an intelligent, data, and advanced analytics-driven feasibility platform.
This platform can help manufacturers systematize study activities, simulate, identify, forecast, and mitigate the risks involved at each clinical trial stage, helping avoid unwarranted setbacks during clinical trials, ultimately leading to successful and timely completion.
Dr. Rao Teki
Guest Author
Dr. Rao Teki, a Lifesciences/Medical Devices Thought & Digital Transformation leader, has over 30+ years of experience in the Lifesciences, Healthcare, and Medical Devices industry. Dr. Rao has Expertise in High-end consulting to Pharma and Bio-Tech companies , in designing Drug Repurposing Platforms, Scientific Social Media based IT Solutions, and in executing business innovation strategies on RWE, Advanced Analytics, EMR-EDC Integration, Cloud, Social Media and Mobile solutions Clinical Informatics.
Dr. Sarika Vanarse
Managing Consultant, iDEAS- Life Sciences & Medical Devices Domain & Consulting
Dr. Sarika Vanarse is a Consulting Leader with 20+ years of global experience into Pharma R&D and Medical devices business & IT consulting and digital transformation. She has successfully defined strategy & roadmap, business & data architecture, process re-engineering, data driven, analytics and insight-based solutions for large digital transformation programs across Pharma & Device value chain, leveraging NextGen technologies, which has resulted into quantifiable higher business values to the Clients. She has a track record of conceptualizing and designing cloud based, enterprise digital architectures and outcomes driven solutions to meet and exceed the end customer expectations.
Sujay Shivram
Practice Lead
Sujay Shivram has 20 + years of experience on Program and Product Management and Enterprise Architecture consulting in Healthcare, Telecom & Energy domains. His expertise areas includes: Thought Leadership, Strategy & Innovation, IT Program Management, Product vision & detailing, Product development & GTM, Enterprise Architecture & Transformational consulting , Application portfolio Support & Maintenance and Pharma Co-vigilance.
Arindam Roychoudhury
Consultant
Arindam has 5+ years of experience in the IT services and consulting industry. Arindam has been involved in Medical Devices/Lifesciences Domain consulting and business development for the application of New Age technologies such as IoT, Blockchain and Automation in businesses across industries. He has a zest for exploring the latest business trends and technologies and also writing technology and business-related articles.