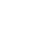How Agentic AI Solves Compliance Challenges
Agentic AI transforms supply chain compliance by directly addressing its core challenges, including complex and evolving QARA requirements coupled with delivery issues, through a consulting-led approach that eliminates inefficiencies and prevents non-compliance at the source.
1. Unified Data Views
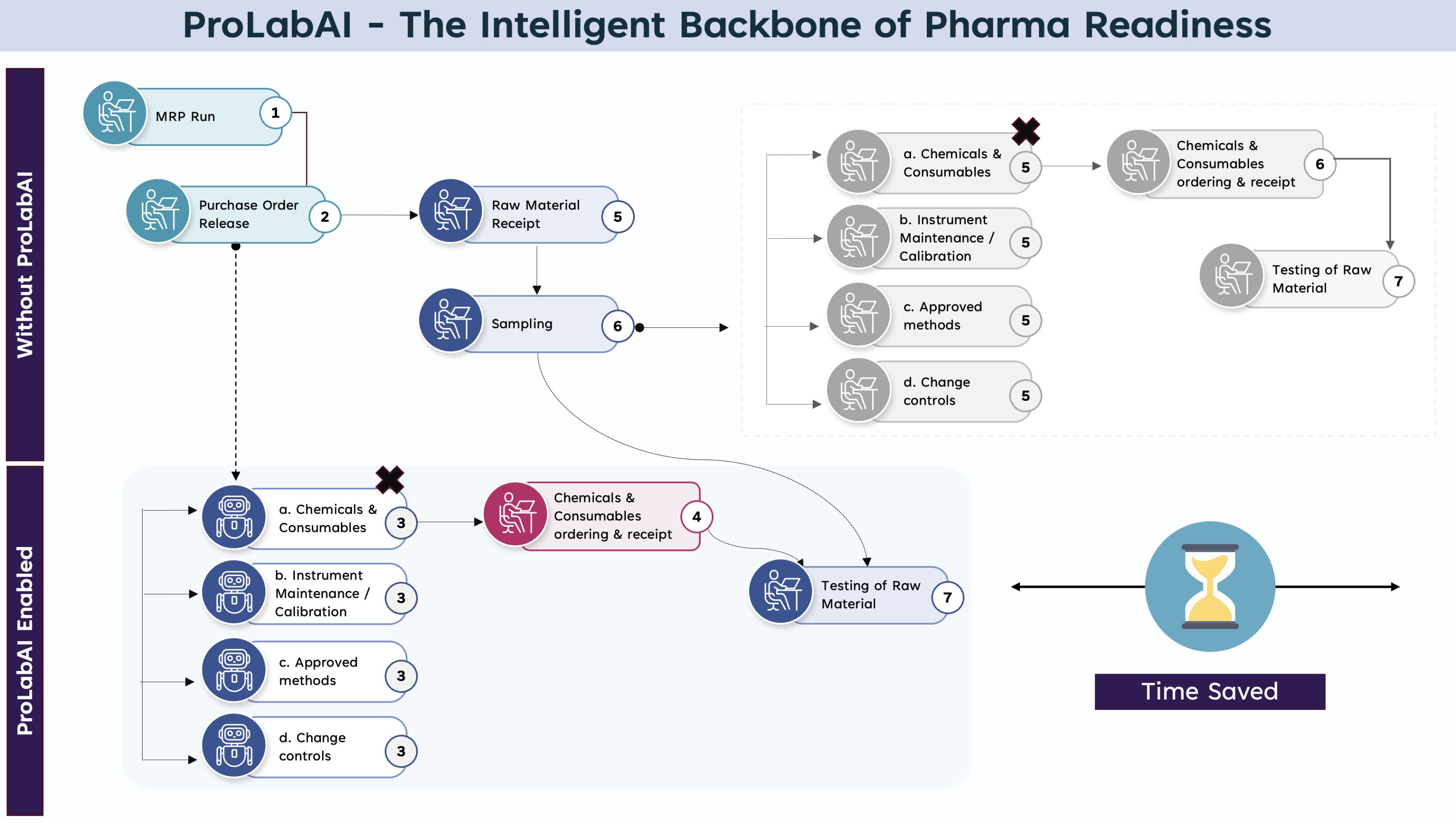
Agentic AI integrates fragmented data spread across disparate systems, such as ERP, LIMS, QMS, and document repositories, into a cohesive and real-time data fabric. This unified view ensures traceability, consistency, and audit-readiness, allowing stakeholders to make informed decisions based on a single source of truth.
2. Real-Time Alerts & Updates
Regulatory landscapes are dynamic, with frequent updates to compliance protocols, documentation standards, and approval timelines. Agentic AI can continuously monitor these changes and delivers real-time alerts to relevant teams. This proactive notification system ensures that compliance is maintained without delay, reducing the risk of missed updates and regulatory breaches.
3. Dynamic Workflow Adaptation
Organizational changes such as mergers, acquisitions, and regulatory shifts can disrupt established workflows. Agentic AI helps navigate these disruptions by analyzing workflow breakdowns, mapping new requirements to existing operations, and proposing adaptive changes, with agents handling 80–90% of the work. It ensures human involvement for validation and oversight where necessary. This intelligent approach minimizes downtime and accelerates cross-functional adaptation.
4. Automation of Routine Tasks
Many manual compliance tasks such as document validation, specification classification, and audit preparation are time-consuming and error prone. Agentic AI automates these activities with precision, reducing human effort and eliminating inconsistencies. This not only improves operational throughput but also enhances the reliability of compliance documentation.
5. Vendor Monitoring and Alignment
Agentic AI monitors external vendor and partner activities by integrating with shared platforms, analyzing documentation and timelines using NLP, and flagging deviations from regulatory norms in real time. This early detection enables corrective actions before issues escalate into compliance risks.